- Related:
Don't forget the left heart
Left heart failure is the most common cause of right ventricular failure.
- Right heart failure can be broken down into “buckets”: too much preload, too much afterload, and/or too little contractility.
- Volume overload (“too much preload”)
- Acute volume overload most commonly is in the setting of something like acute TR (e.g. due to endocarditis)
- Chronic excessive RV preload can occur in the context of
- right-side (tricuspid or pulmonary) valvular insufficiency
- intracardiac left-to-right shunts (see Intracardiac Shunts)
- e.g. ASD, VSD, sinus venosus defect, coronary sinus defect, PAPVR
- extracardiac arteriovenous shunts
- ⚠️ the creation of AV fistulas or grafts leads to chronic RV dilatation and reduced RV systolic function
- Pressure overload (“too much afterload”)
- Acute increases in afterload (e.g. acute PE) are poorly tolerated by the RV.
- Acute lung injury diagnosed on the basis of lung imaging may also cause rapid and acute increases in the RV afterload and may be further exacerbated by the impact of positive pressure ventilation.
- Chronic increases in afterload occur with things like pulmonary hypertension, CTEPH, PS, chronic hypoxemia, and longstanding elevated LAP causing group 2 PH (MR/MS, HFrEF, HFpEF).
- Acute increases in afterload (e.g. acute PE) are poorly tolerated by the RV.
- Primary cardiomyopathic processes (i.e., ischemia, infiltration, ARVC) - “too little contractility”
- causes include cardiac amyloidosis, RV infarction, post-transplant RV dysfunction, and ARVC
- Cardiac sarcoidosis may also be manifested as predominant right ventricular dysfunction and may mimic ARVC.
- Acute: MI, myocarditis, cardiac surgery
- In most patients, the majority of the RV’s blood supply comes from the RCA through the RV marginal branches. ∴, proximal RCA occlusion can substantially compromise RV contractility.
- causes include cardiac amyloidosis, RV infarction, post-transplant RV dysfunction, and ARVC
- Volume overload (“too much preload”)
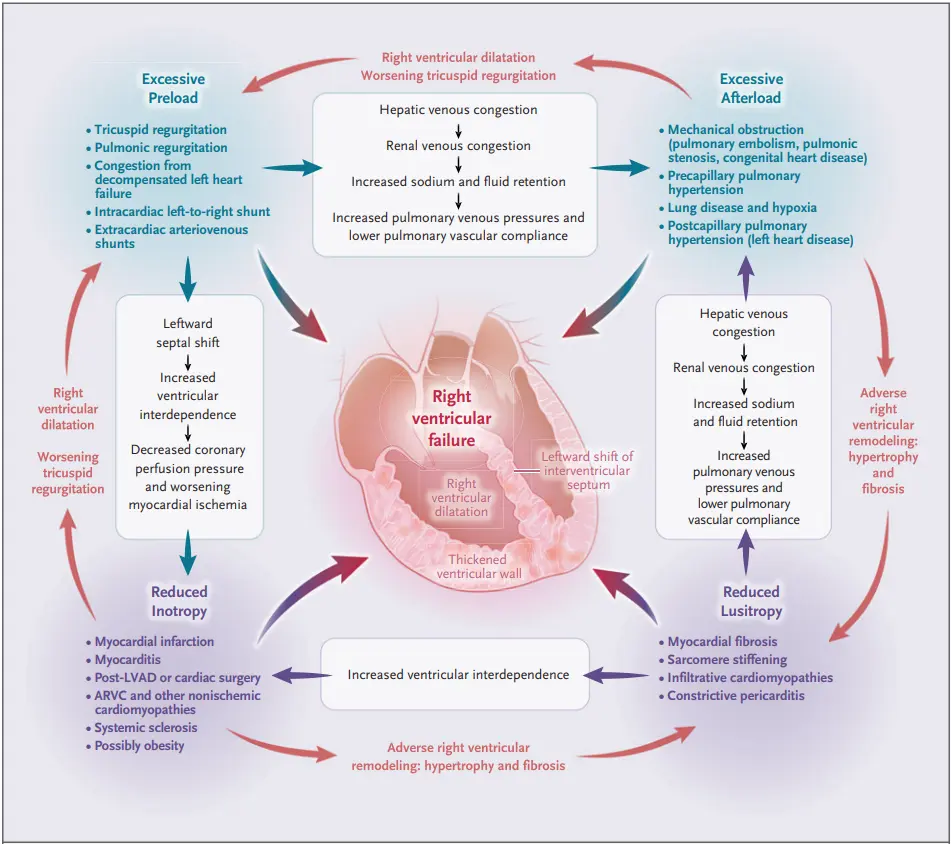 Figure source: 1
Figure source: 1
- 💡 In cases of severe right ventricular enlargement and dysfunction without apparent cause, look for a left to right shunt lesion (i.e., VSD, ASD, PAPVR). Sometimes further imaging (TEE, cardiac CT, cardiac MRI) is necessary to detect these lesions if not visualized on TTE.
- Sinus venosus defect
- RHC for characterizing shunt defects (Source: https://www.cardionerds.com/106-case-report-a-hole-in-the-hfpef-diagnosis-boston-university-massachusetts-general-hospital-and-brigham-and-womens-hospital/)
- A left-to-right shunt is when oxygenated blood from the systemic circulation (left) inappropriately mixes with the pulmonary circulation (right), increasing the oxygen concentration. This can occur via anomalous pulmonary veins, defects at the atrial or ventricular level, or sometimes systemic arterio-venous fistulas.
- As the SGC is passed through the great vessels and cardiac chambers, samples are collected at various levels.
- A left to right shunt is detected by an oximetry “step up” where oxygenated blood from the systemic circulation blood mixes with deoxygenated blood from the venous circulation.
- An oxygen saturation step up of >7% is considered significant at the level of the great veins and RA while a step up of >5% is considered significant at levels distal to the RA.
- Qp/Qs
- Calculated to examine the degree of left to right shunting
- Small shunts are defined by Qp/Qs <1.5.
- These are often asymptomatic and generally do not need to be treated.
- Large shunts are defined by Qp/Qs >2 and often require closure.
- Interpretation: for a Qp/Qs = 3.1, it means that for every 1 L of cardiac output through the systemic circulation, 3.1 L are going through the pulmonary circulation
- Because intra-cardiac shunts will affect the mixed venous (pulmonary artery oxygen saturation), a systemic mixed venous saturation needs to be calculated to estimate “pre-shunt” mixed venous O2. This is defined by Flamm’s formula:
Diagnosis
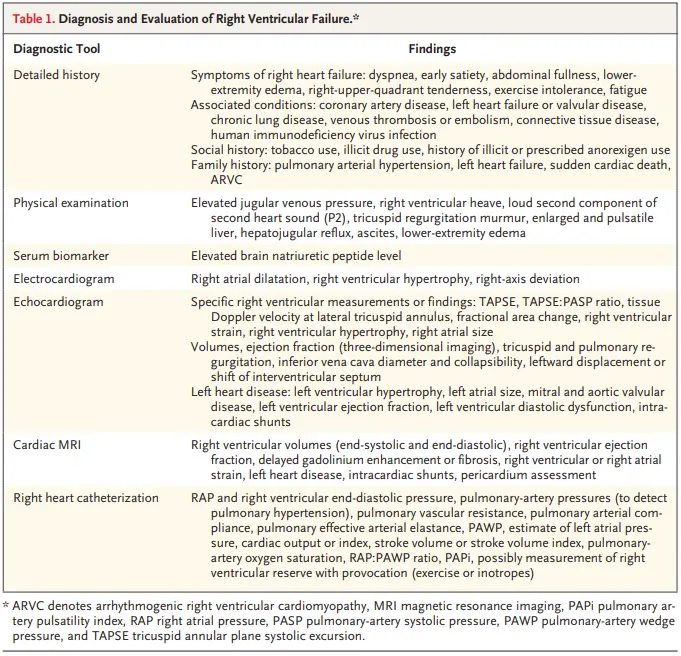 Figure source: 1
Figure source: 1
History
- Symptoms include dyspnea, LE edema, early satiety, abdominal fullness, fatigue, exertional intolerance, and RUQ tenderness.1
Exam
- Exam findings may include elevated JVP, RV heave on palpation, a prominent pulmonic component of the second heart sound (P2; indicative of PH), murmur of TR, palpable and pulsatile liver (indicative of clinically significant TR), hepatojugular reflux, ascites, and LE edema.1
Cardiac MRI
- 🥇 Cardiac MRI is the reference standard for assessment of right ventricular size, right ventricular ejection fraction, and mass.
Echo
- Recall, RV systolic function can be assessed by:
- 🌟 tricuspid annular plane systolic excursion (TAPSE)
- RV fractional area change
- free-wall strain
- tricuspid annular systolic mitral annular tissue velocity (s′)
- RV index of myocardial performance
- TAPSE
- TAPSE/PASP ratio
- 📝 A dilated or thickened RV and dilated RA suggest a chronic state of RV dysfunction.1
- Septal flattening
- Flattening of the septum during diastole indicates volume overload, whereas flattening in systole is seen in pressure overload states.
- Leftward septal displacement can be quantified as the eccentricity index, the ratio of the anteroposterior dimension to the septolateral dimension.1
- Eccentricity index >1 indicates RV overload
- Dilatation of the IVC without inspiratory collapse indicates elevated right atrial pressure.
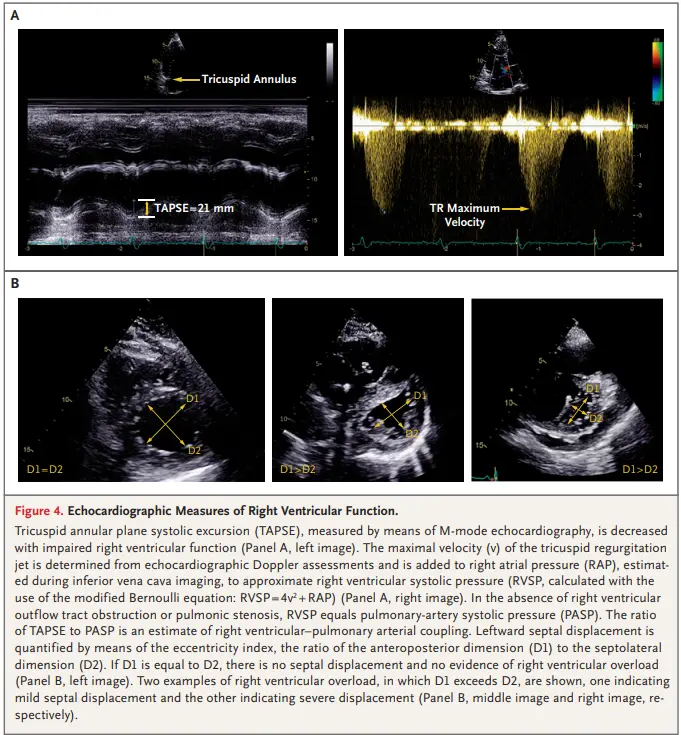 Figure source: 1
Figure source: 1
Invasive Hemodynamics (RHC)
- Measures of Preload
- RAP
- RVEDP
- Measures of Afterload
- PVR, PA compliance, PA elastance
- Other surrogate measures
- SV, SV indexed
- RAP/PCWP
- RV stroke work index
- Pulmonary artery pulsatility index (PAPi)
Treatment
- Recall that the mechanisms can be too much preload, too much afterload, and/or too little contractility. Naturally, treatment will involve a focus on optimization of preload, reduction of RV afterload, and augmentation of RV contractility.
- Treat the underlying cause, e.g. revascularization of acute RV MI, thrombectomy/AC for the PE, immunosuppression if d/t inflammatory heart disease, etc.
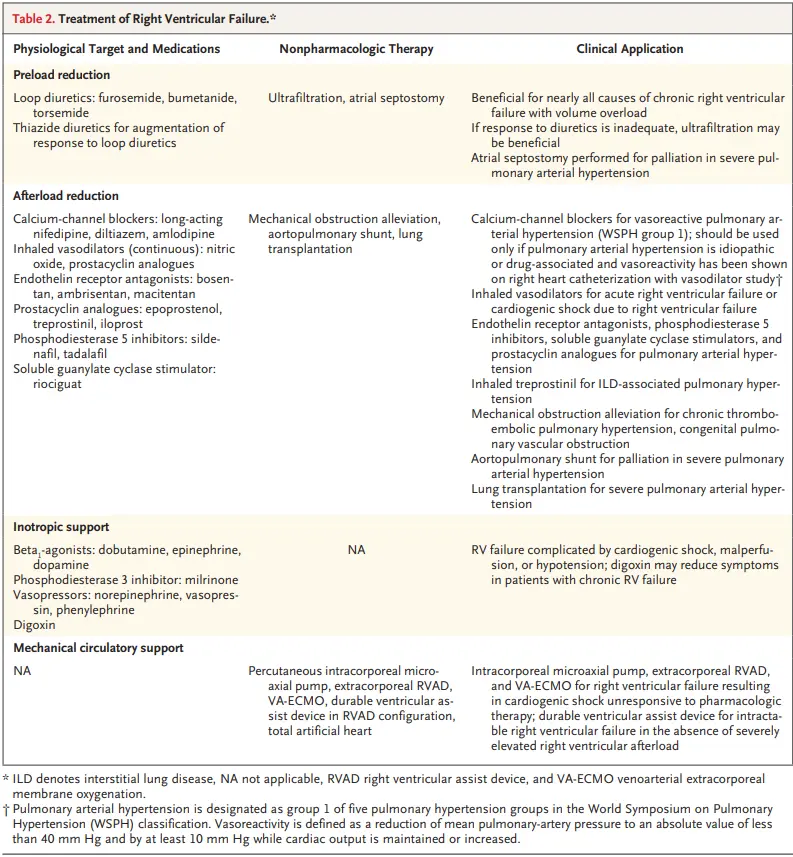 Figure source: 1
Figure source: 1
Treat Preload
- Fluids - ⚠️ not for all patients with RV failure!!!
- Useful in cases where patient is in shock because of an acute increase in RV afterload (due to acute PE) or an acute reduction in contractility (due to a RV infarct) may benefit from volume loading to augment RV stroke volume and increase transpulmonary blood transit.1
- Diuresis
- Patients with chronic RV failure benefit from volume removal and decongestion.1
- Normalization of the preload can reduce TVannular dilatation and tricuspid regurgitation, RV wall stress, and septal deformation.
- Volume reduction is most commonly accomplished with IV diuretics, though ultrafiltration may be necessary in some cases.
- Patients with chronic RV failure benefit from volume removal and decongestion.1
Treat Afterload
- Left Heart Failure
- Reduction of RV afterload in patients with left heart disease and persistent pulmonary hypertension is best achieved through optimization of GDMT and normalization of left-heart filling pressures.
- Volume removal in patients who have hypervolemia with RV failure in the setting of left heart disease will improve RV afterload + preload.
- Pulmonary Arterial Hypertension (PAH)
- For patients with acute RV failure and chronically elevated precapillary RV afterload, inhaled pulmonary vasodilators (nitric oxide or epoprostenol) can provide immediate improvement.
- CTEPH
- In patients with pulmonary hypertension due to chronic thromboembolic disease, surgical pulmonary endarterectomy or (in inoperable patients) percutaneous balloon pulmonary angioplasty should be considered, alongside anticoagulation therapy.
- Sleep apnea
- treat the sleep apnea
Augment Contractility
- Inotropic or vasodilator support may be necessary to maintain cardiac output.
- Inotropes
- Dobutamine - increases CO and SV in patients with RV MI and those with PH
- Milrinone - careful attention to avoid excess systemic vasodilatation and hypOtension; may lead to RV ischemia and ↓ in LV contractility
- Digoxin - mixed results
- RV failure + Shock refractory to pharmacologic therapy
- Avoid temporary isolated RVAD should not be used due to predisposition to pulmonary hemorrhage