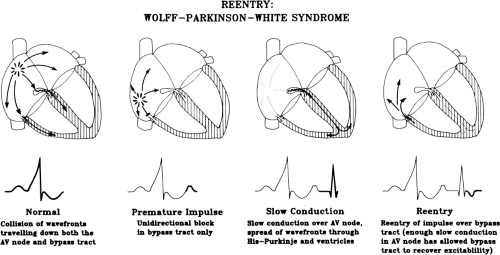
- The 3 main mechanisms for tachyarrhythmias are:
- Initial approach to analyzing a tachycardia:
- Determine the ventricular rate
- Tachyarrhythmias refer to a ventricular rate > 100 bpm
- Regular vs Irregular ventricular rate
- What are the ventricles doing? Narrow QRS vs wide QRS (≥ 120 ms)
- use the lead with the widest QRS
- If wide → look for AV dissociation and the number of P waves compared to number of QRS complexes
- If narrow → assess the length of the RP interval
- What are the atria doing? study the P waves, sinus rhythm?
- What is the AV junction doing? P wave and QRS relationship, PR-interval
- P waves normally have consistent morphology and timing (can be marched out)
- Determine the ventricular rate
- There are 3 major mechanisms in Tachyarrhythmias:
- Re-entry (MCC)
- Re-entry represents an anatomical electric circuit which allows for the maintenance of an arrhythmia under these 3 conditions:
- A trigger (i.e.. Premature Atrial Complexes (PACs))
- A functional electric circuit
- Two arms of the electric circuit with different electrophysiologic properties
- Re-entry represents an anatomical electric circuit which allows for the maintenance of an arrhythmia under these 3 conditions:
- Automaticity
- Triggered activity
- Re-entry (MCC)
- Rates of different tachyarrhythmias:
- 150-250 per minute → Paroxysmal Tachycardia
- 250-350 per minute → Flutter
- 350-450 per minute → Fibrillation
- Determine origin
- A tachyarrhythmia is easily recognized by rate alone, but the specific diagnosis requires that we identify the origin, i.e. we must determine the location of the irritable automaticity focus (atrial, junctional, or ventricular).
- For select arrhythmias known to have a high cure rate with ablation therapy (e.g., atrial flutter, paroxysmal supraventricular tachycardia, WPW syndrome, idiopathic premature ventricular complexes/ventricular tachycardia), catheter ablation may be indicated as a first-line treatment.
- Can classify by origin or by chamber. But Dr. Gupta recommends thinking about it mechanistically
- 3 mechanisms
- Enhanced normal automaticity
- Reentry
- Triggered activity
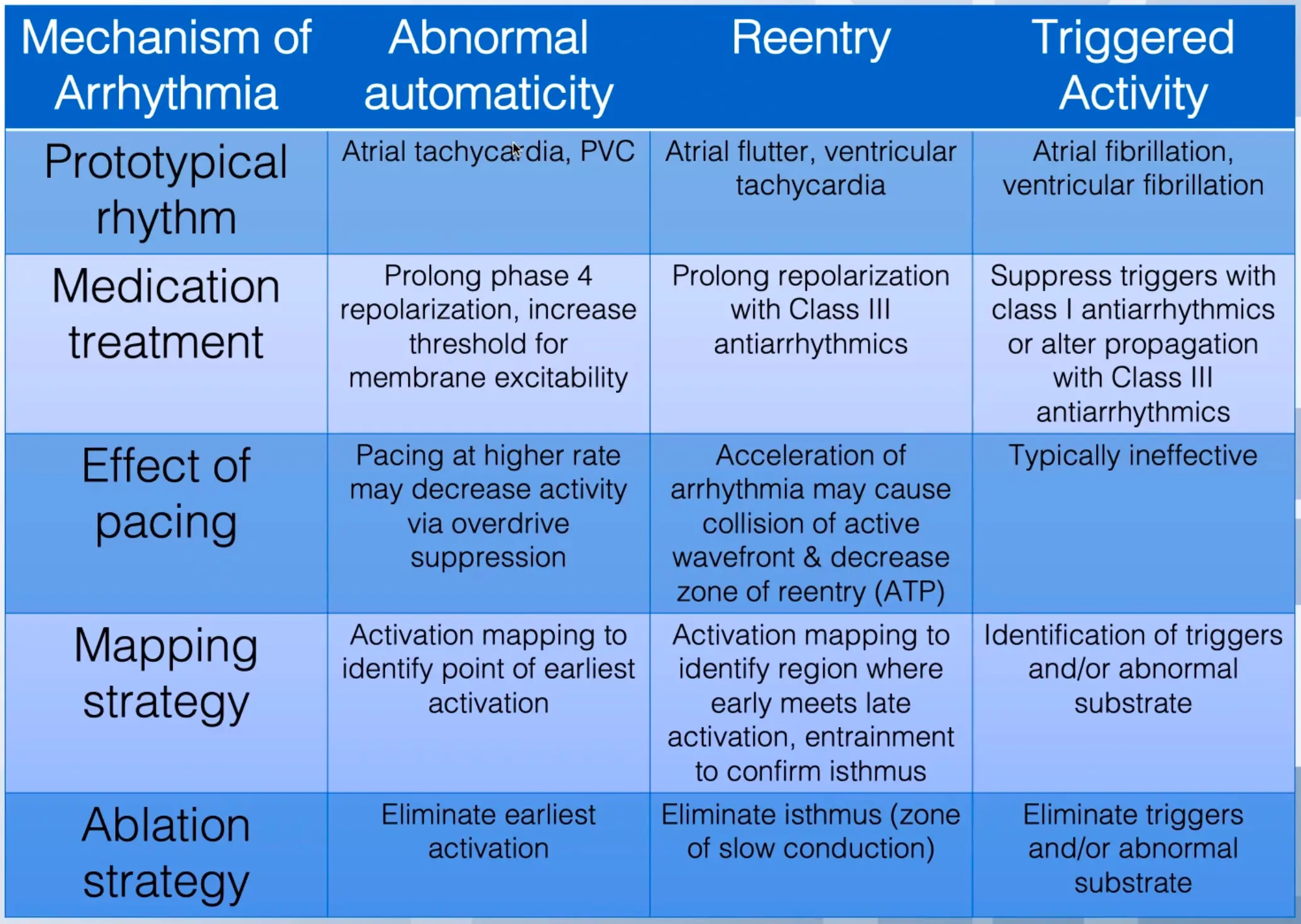
| Tachycardia | Mechanism | Origin | AV or VA Conduction |
|---|---|---|---|
| Sinus Tachycardia | Automatic (normal) | Sinus node | 1:1 |
| Sinus node reentry | Reentry | Sinus node and right atrium | 1:1 or variable |
| Atrial Fibrillation (AFib) | Reentry, automatic, triggered activity | Atria, thoracic veins, pulmonary veins, SVC, vein of Marshall | Variable |
| Atrial flutter | Reentry | RA, LA (infrequent) | Variable |
| Atrial tachycardia | Reentry, automatic, triggered activity | Atria | 1:1, 2:1, or variable |
| AV nodal reentry tachycardia | Reentry | AV junction | 1:1 or variable |
| AV reentry (WPW or concealed accessory AV connection) | Reentry | Circuit includes accessory AV connection, atria, AV node, His-Purkinje system, ventricles | 1:1 |
| Accelerated AV junctional tachycardia | Automatic | AV junction (AV node and His bundle) | 1:1 or variable |
| Accelerated idioventricular rhythm | Abnormal automaticity | Purkinje fibers | Variable, 1:1, or AV dissociation |
| Ventricular tachycardia | Reentry, automatic, triggered | Ventricles | AV dissociation, variable |
| Bundle branch reentrant tachycardia | Reentry | Bundle branches and ventricular septum | AV dissociation, variable, or 1:1 |
| RVOT | Automatic, triggered activity | RVOT | AV dissociation, variable, or 1:1 |
| TdP tachycardia | Reentry, triggered activity | Ventricles | AV dissociation |
| Bidirectional tachycardia | Triggered activity | Purkinje cells | AV dissociation |
| Table Source |
Wide-Complex Tachycardia
Narrow Complex Tachycardia
See Supraventricular Tachycardia (SVT)
Enhanced normal automaticity
Automaticity (<10% of abnormal tachyarrhythmias) arises when you have abnormal acceleration of phase 4 activity at some location of the heart. This abnormal automatic focus can appear in the SA node, atria, AV junction, or the ventricles (thus leading to automatic atrial, junctional, or ventricular tachycardia, respectively). 1
- Good ol’ normal automaticity results from phase 4 spontaneous depolarization of the transmembrane action potential arising from a normal resting potential, reaching threshold and initiating an action potential.2
- By contrast, abnormal automaticity arises from a partially depolarized membrane potential that is usually close to the activation potential for calcium channels in the cell membrane.2
- Prototypical rhythms are atrial tachycardia, inappropriate sinus tachycardia, premature ventricular contractions, premature atrial contractions, junctional tachycardia
- Automatic tachyarrhythmias often display a warm up and warm down rate when the arrhythmia begins and ends.1
- Causes include sinus tachycardia, metabolic causes (ischemia, hypoxemia, hypokalemia, hypomagnesemia, acid-base disorders, increased sympathetic tone, use of sympathomimetic agents).1
- For example, acute pulmonary disease can lead to multifocal atrial tachycardia, which is the most common type of automatic atrial tachycardia.
- Automatic arrhythmias should be treated primarily by identifying and reversing the underlying metabolic cause.1
- In the acute phase of an MI or during transient ischemia, increased extracellular potassium causes partial depolarization of the resting membrane potential creating injury currents between the infarcted/ischemic tissue and healthy myocardium. These injury currents may initiate spontaneous activity.2
- Automatic tachyarrhythmias cannot be induced by programmed pacing techniques, so these arrhythmias are generally not amenable to provocative study in the EP lab. 1
- Sinus node and AV node are missing phases 1 and 2, i.e. only Phases 0, 3, and 4.
- Different from other myocytes
- Depol - Ca influx
- Repol - K eflux
- ↓ Na influx (Phase 4) is reflected as slower slope; prolonged repol at SA node → bradycardia
- e.g. ↑ ACh with parasympathetic stimulation → ↓ slope of Phase 4 → brady
- ↑ Na influx (Phase 4) is reflected as increased slope → tachycardia
- e.g. ↑ NE with sympathetic stimulation → ↑ slope of Phase 4 → tachy
- CCBs slow the rate of action potential rise → slows depolarization of Phase 3(?)
- e.g. Verapamil sensitive PVCs
- Na channel blockers raise the action potential threshold for AP firing
- e.g. use of these meds for inappropriate tachy/junctional tachy


Re-entry
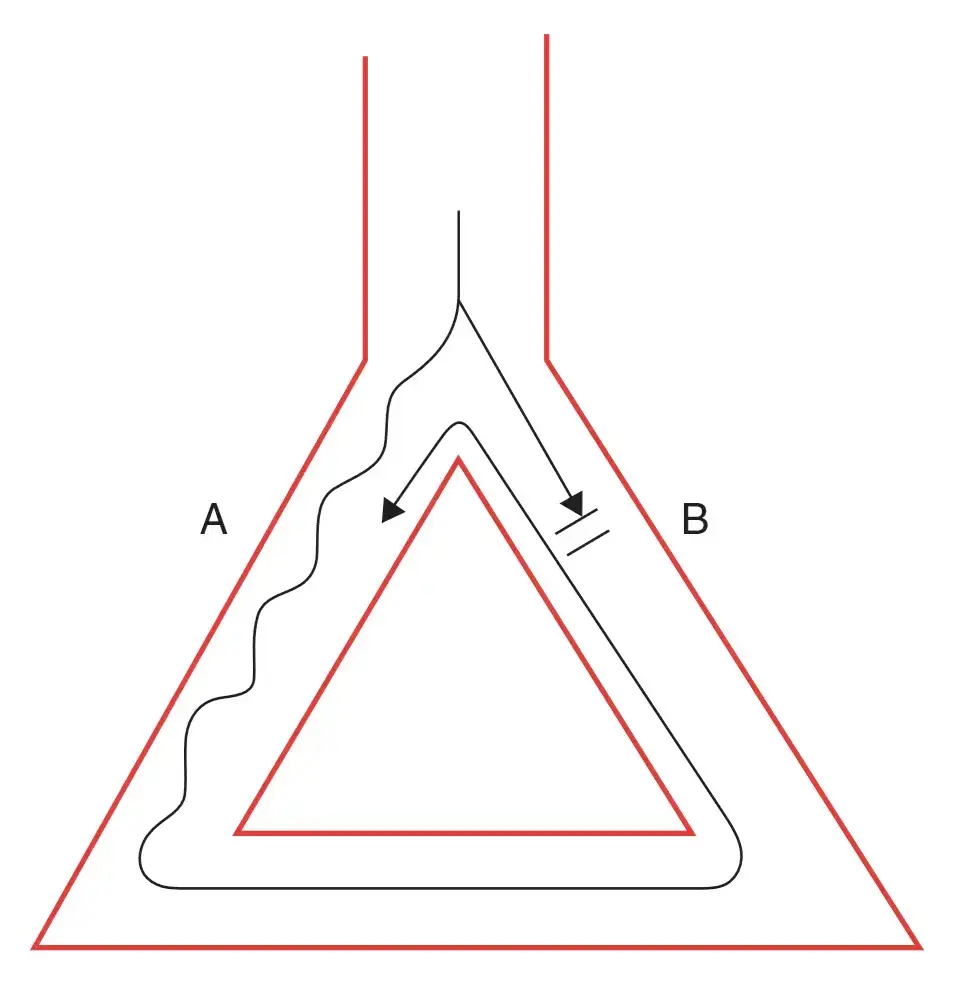 A core assumption here is that pathway A conducts slower, and has a shorter refractory period. Pathway B conducts faster, but has a longer refractory period. Recall, Dr. Gupta’s boat-and-wake analogy from AV Nodal Reentrant Tachycardia (AVNRT) where the fast boat creates a long wake (long refractory period) and the slow boat has a short wake. A signal that originates at the top of the diagram (above the branch) may conduct through both pathways. The signal conducted through A may conduct into B (e.g. if B is blocked → not in refractory period), which then continues conducting all the way through A again, creating a perpetual loop.
A core assumption here is that pathway A conducts slower, and has a shorter refractory period. Pathway B conducts faster, but has a longer refractory period. Recall, Dr. Gupta’s boat-and-wake analogy from AV Nodal Reentrant Tachycardia (AVNRT) where the fast boat creates a long wake (long refractory period) and the slow boat has a short wake. A signal that originates at the top of the diagram (above the branch) may conduct through both pathways. The signal conducted through A may conduct into B (e.g. if B is blocked → not in refractory period), which then continues conducting all the way through A again, creating a perpetual loop.
- Reentry is the most common mechanism for tachyarrhythmias, 1 especially sustained ventricular arrhythmias in the presence of structural heart disease.2
- Easy to study in the EP lab.1
- Reentrant arrhythmias are ideal for study in the EP lab because they can be reproducibly induced and terminated with appropriately timed impulses → by inducing reentrant arrhythmias in a controlled setting, the location of the anatomic circuit can be mapped and the effect of various therapies assessed.
- Just as reentry can be initiated by premature beats, it can be terminated by premature beats.1
- Reentry depends on differences in conduction velocities and refractory periods in the different pathways of the reentrant circuit.1 For example, in the figure below, pathway A and B have different refractory periods and different conduction velocities, separated by an anatomical or functional barrier. (Source)
- The pathway with the shorter refractory period needs to have a slower conduction.
- The larger the difference in refractory period between A and B, the easier it is to initiate an arrhythmia; this difference between refractory periods is called the tachycardia zone.
- The slower the conduction velocity in A, the more likely it is to sustain the arrhythmia (slow conduction leads to a large excitable gap
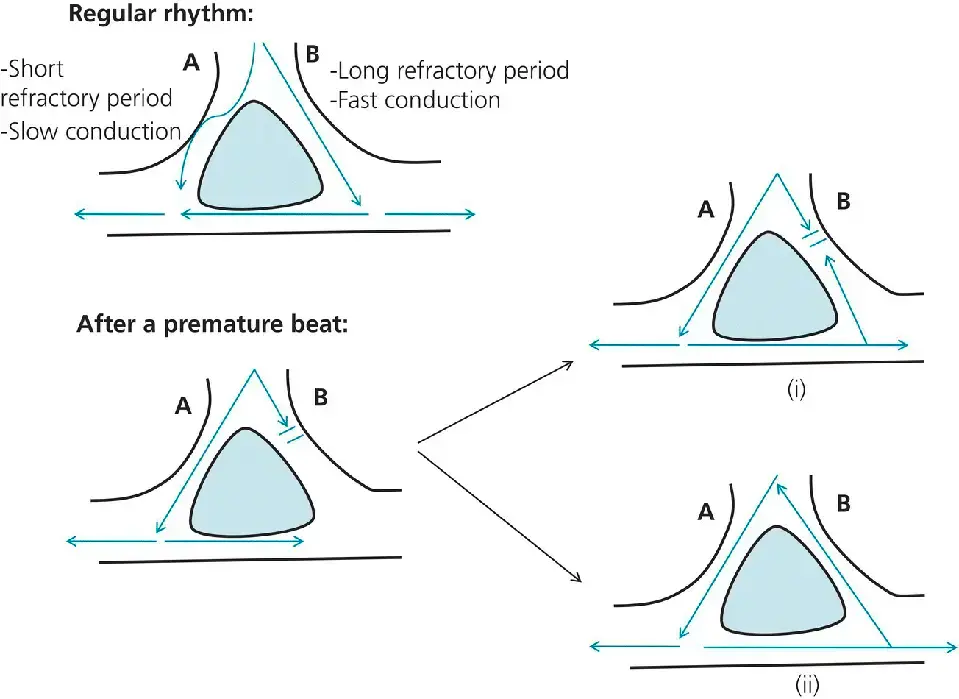
- When the rhythm is regular, the electrical activity spreads through both areas simultaneously (top).
- After a premature beat, region B is still in its long refractory period, but region A has recovered (lower left). The electrical activity spreads through region A, then meets the tail end of region B; at that time, if region B has not recovered from its refractory period, arrhythmia does not occur (i). Since A conducts slowly, region B may well recover from its refractory period, allowing conduction to spread retrogradely through region B (ii). Eventually, this retrograde impulse reaches A faster than the electrical activity originating from the sinus node reaches A. Since A has a short refractory period, the impulse may propagate through A and stimulate the whole cardiac chamber again. Thus, this circle keeps repeating itself, stimulating the myocardial tissue at a rate faster than normal: this is the reentrant tachyarrhythmia.
- The existence of structural reentrant substrates provide the rationale for VT ablation in scar-related VTs.
- Once depol, needs enough time to repol.
- Excitable gap: region of tissue
NOTE
Anti-tachycardia pacing makes you go faster in an effort to help to ↓ the excitable gap.
- Requirements
- Central area of block and critical tissue mass
- Unidirectional conduction block
- only goes in 1 direction
- Area of slow conduction
- area where slows down for excitable gap to occur
- Initiating trigger
- something to start off the rhythm
- Treating reentrant arrhythmia sometimes involves trying to narrow or abolish the tachycardia zone (by increasing the refractory period of pathway A or decreasing the refractory period of pathway B in the example shown).1
- WPW
- Re-entry in Ventricular Tachycardia (VT)
- Myocardial ischemia creates scar tissue, creating a region of unexcitable tissue as well as surrounding tissue with unidirectional conduction
- AV Nodal Reentrant Tachycardia (AVNRT)
Triggered Activity
-
Triggered activity has some features of both automaticity and reentry.
-
Like automaticity, triggered activity involves a leakage of positive ions into the cardiac cell, leading to a bump in the Action Potential in late phase 3 or early phase 4 (often called an afterdepolarization). If these afterdepolarizations are of sufficient magnitude to engage the rapid sodium channels (i.e. if they reset the threshold voltage), another action potential can be generated.1
- 📝 tl;dr: Triggered activity is like automaticity in that new action potentials can be generated by leakage of positive ions into the cell. Unlike automaticity, triggered activity is not always spontaneous (therefore not truly automatic) and can be provoked by premature beats (kind of like with reentry).
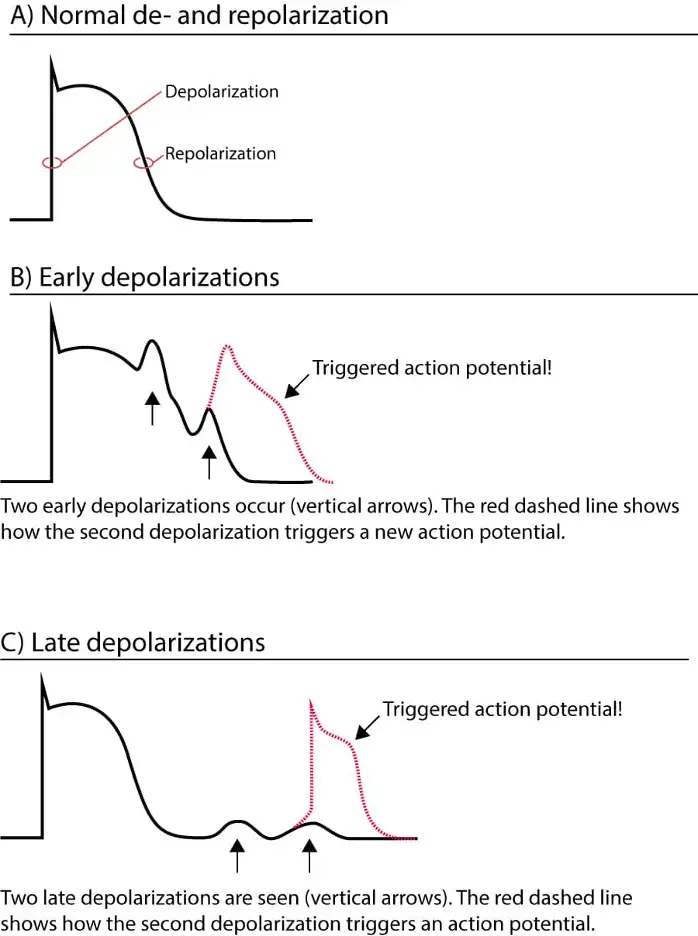
-
Triggered activity resembles automaticity and displaying warm up and warm down. 1
-
Triggered activity is felt to depend on calcium channel send thus may respond to CCBs.
-
Prototypical rhythms are Torsades de Pointes, polymorphic ventricular tachycardia, ventricular fibrillation, AFib
-
Caused by EADs, DADs or tissue level hypoxia (ischemia, pH changes).
-
Treated with medications that reduce membrane excitability, such as class I antiarrhythmics; can also use class Ill antiarrhythmics to slow propagation
-
Pacing typically ineffective
-
Ablation to eliminate focus can be effective - if a single or limited set of foci can be identified.
-
This involves both a trigger to set off the rhythm and substrate to maintain the rhythm.
-
The protypical examples is atrial fibrillation with originates within the pulmonary veins of the left atrium and is the perpetuated throughout both atria.
-
The typical mechanisms are early after depolarizations (EADs) and delayed after depolarizations (DAD)
-
Mechanisms table from Gupta slides has nice comparison b/w the 2
-
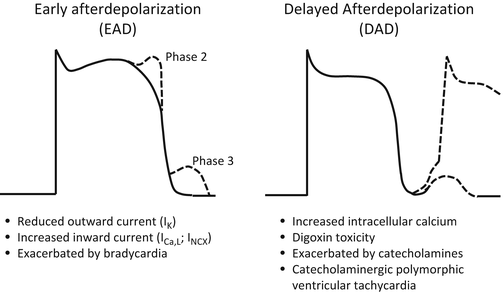
-
R on T phenomenon is just an EAD
| EADs | DADs | |
|---|---|---|
| Inciters | QT prolonging 💊 | Digoxin Catecholamines |
| Exaggerated by | Bradycardia HypOkalemia | Tachycardia |
| Blunted by | Tachycardia Magnesium/Potassium supplementation Isoproterenol | CCBs BBs Adenosine |
| Mechanisms | ↑ net inward plateau current | Intracellular Ca overload (leaky RYR2 channels) |
| Putative clinical rhythm | Torsades de Pointes | CPVT ARVC Ischemia Digoxin toxicity |
Early afterdepolarizations (EADs)
- Bradycardia-dependent
- Occurs in phase 2 or early phase 3 repolarization
- usually in the setting of action potential prolongation due to an increase in inward currents (the late sodium current, the inward calcium current or the sodium calcium exchange current) or a decrease in repolarizing potassium currents
- Example: brady, hypoK, hypoMg exaggerate Phase 3 → more opportunity for EADs
- Responsible for mechanism of TdP with or without Long QT Syndrome
- Inhibited by tachypacing (↑ HR shortens depolorization), magnesium (recall, cofactor for K channels), correction of hypOkalemia
- If you shorten QT interval, then the EAD will just be a PVC. Trying to avoid R on T and triggering TdP (Brady-induced TdP)
- Under these conditions, EADs may be initiated when reactivation of the inward L-type calcium channel occurs before the membrane has returned to a more negative potential than that required for calcium channel reactivation. Spontaneous calcium release from the sarcoplasmic reticulum may also result in activation of a depolarizing sodium/calcium exchange current.
- EADs are the trigger for TdP associated with QT prolongation either induced by medications or other acquired factors or due to mutations of ion channels causing Long QT Syndrome. In these cases, it is possible that the EAD/triggered activity sequence is the trigger that culminates in polymorphic VT/VF.2
Delayed afterdepolarizations (DADs)
- Tachycardia-dependent
- Tachycardia → calcium overload, which increases generation of DADs to occur → spontaneous depolarization. ∴ treatment by inducing bradycardia and/or correction of hypercalcemia to reduce overall Ca concentration
- Central mechanism involves calcium overload
- Factors contributing to elevated intracellular calcium load include tachycardia, catecholamines, hypokalemia, digoxin toxicity, cardiac hypertrophy, and HF. Elevated sarcoplasmic calcium content or increased sensitivity of the ryanodine receptor can initiate spontaneous calcium release, which activates a transient inward current driven predominantly by the sodium-calcium exchange current.
- If the membrane depolarization is sufficiently large, the inward sodium current is activated resulting in a triggered action potential.
- Example: calcium overload → recurrent premature beats (can’t get the Ca back in the cell → recurrent activity) → DADs
- Responsible for mechanism for RVOT VT, reperfusion arrhythmias, Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), digitalis toxicity
- Treated with CCBs (makes sense as mechanism is d/t Ca overload), BBs, Adenosine
- Occur after complete membrane repolarization and develop under conditions of intracellular calcium overload. DADs are the underlying mechanism for VT in the setting of digoxin toxicity, CPVT, and idiopathic outflow tract VA. DADs are also considered to be an important trigger of VA in the setting of HF. Purkinje cells are more susceptible to spontaneous sarcoplasmic reticulum calcium release than ventricular myocytes suggesting that delayed afterdepolarizations may be an important mechanism for some Purkinje fiber-related VA.2
Questions
A 81 y/o woman presents with recurrent episodes of palpitations associated with fatigue, dyspnea but no chest pain. Episodes have been recurrent and increasing in frequency over months. She takes no medications. Her ECG is shown:
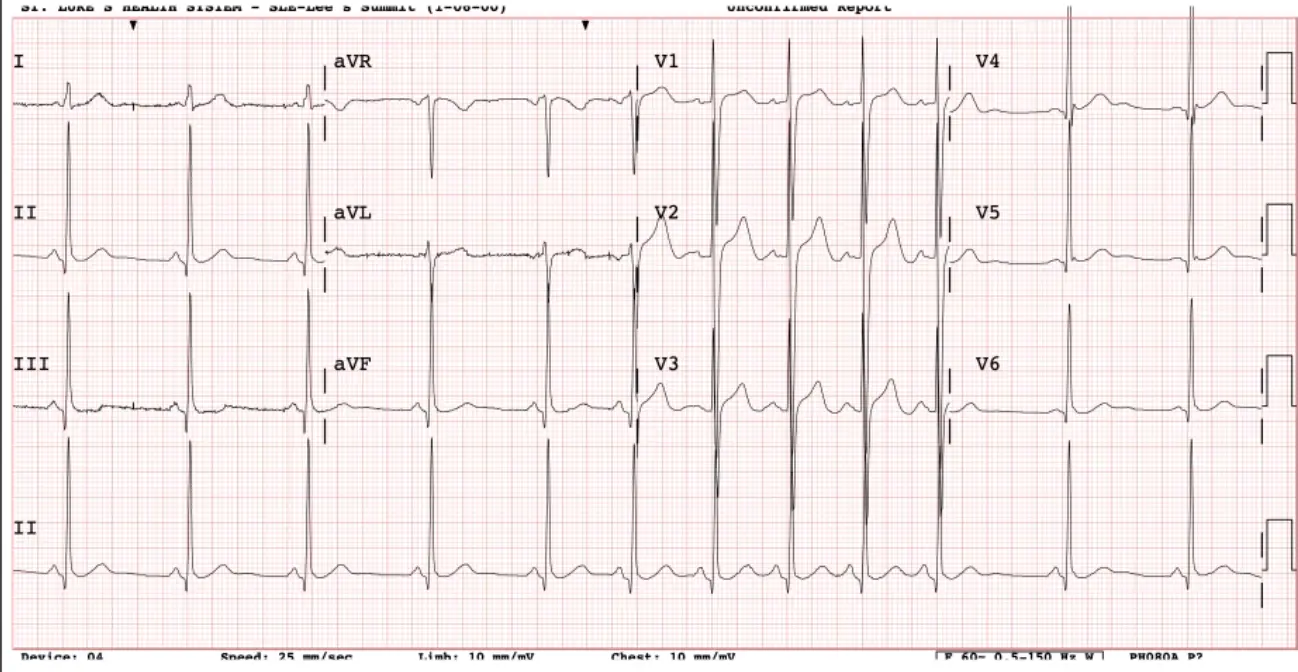
Looks like atrial tachycardia (which is abnormal automaticity). 1 spot in her atria with high slope/phase 4 going too fast, firing on its own
What is the most appropriate initial therapy for her? A. ==Diltiazem== B. Flecainide C. Catheter ablation D. Nothing, routine follow up
If she comes back with constipation, etc. (not tolerating), then maybe we can try flecainide. If you’re treating PACs, PVCs, Atach, etc. you can use single agent flecainide. Combo of flecainide with diltiazem, etc. is for Atrial Fibrillation (AFib), Atrial Flutter and others.
A 74 y/o woman with a previous history of PAF s/p catheter ablation presents with an elevated heart rate, fatigue and dyspnea. An ECG is shown:
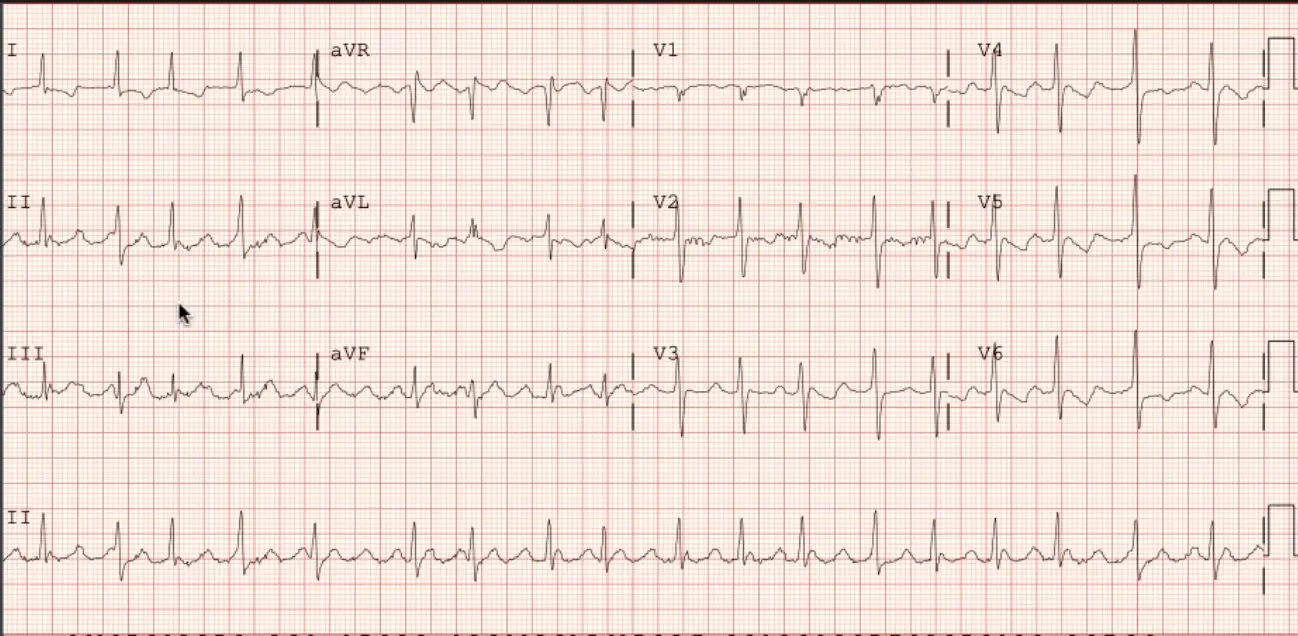
Atypical Atrial Flutter. Atypical because draw line from QRS and it is “positive” because above this line.
Which of the following statements are true regarding the mechanism of this arrhythmia? A. It is an example of abnormal automaticity B. Early after depolarizations (EADs) or Delayed after depolarizations (DADs) are the most likely causes C. Calcium channels blockers are expected to be curative D. ==Anti-tachycardia pacing will likely be effective==
A 76 y/o man presents with symptoms of fatigue, decreased stamina and has this ECG taken. He is very symptomatic and is admitted to the hospital.
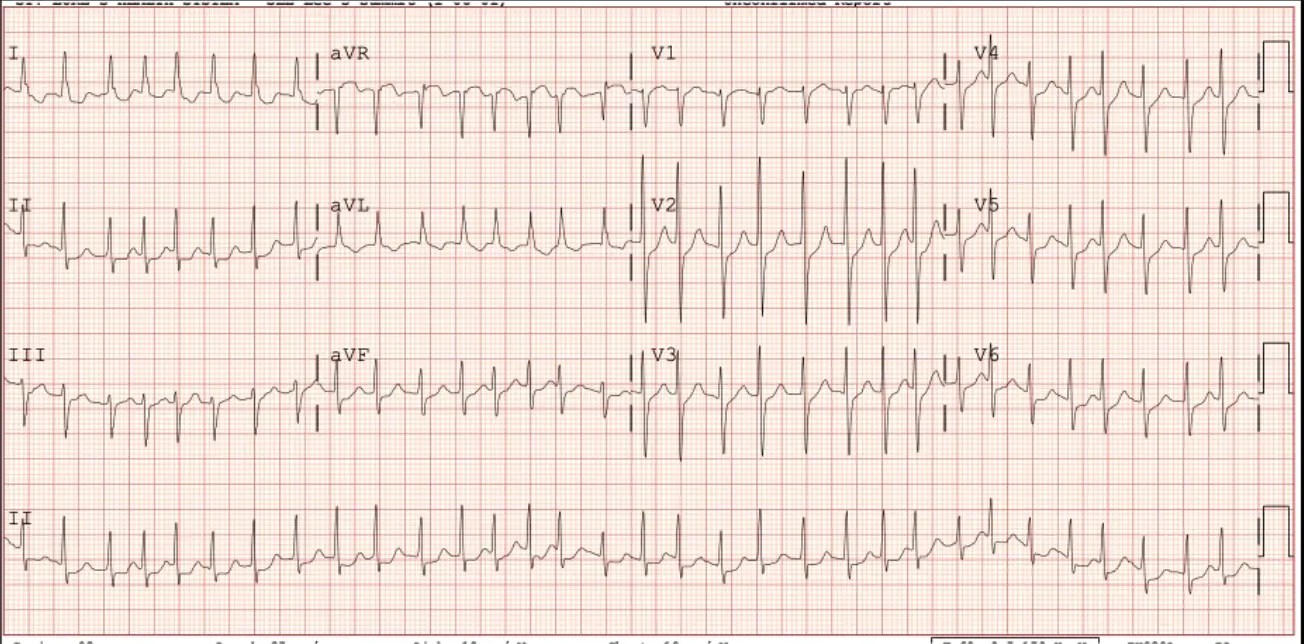
AFib with RVR
Which of the following therapies is LEAST likely to be effective? A. Metoprolol B. Sotalol C. Catheter ablation D. ==Overdrive pacing==
A 56 y/o woman presents with symptoms of fatigue, palpitations and has this ECG taken. She is symptomatic and asking about treatment options
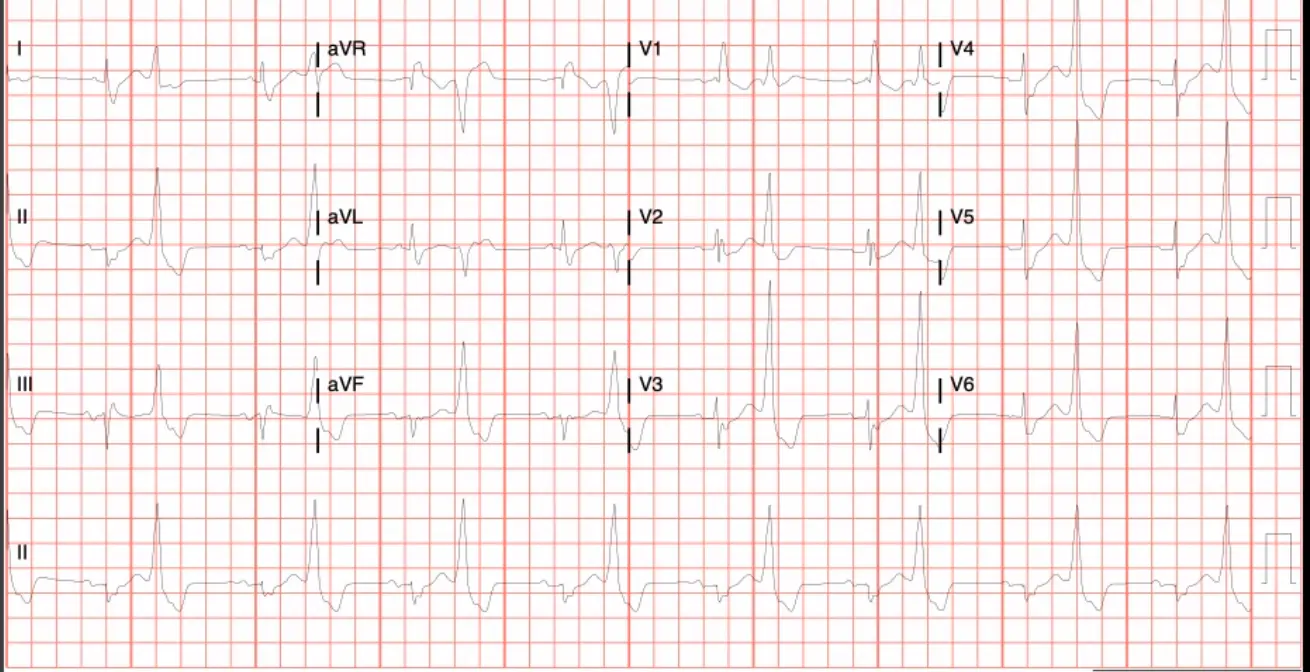
Ventricular bigeminy, so the abnormal automaticity mechanism is in play. DADs causing PVCs to occur?
Which of the following statements are true regarding this arrhythmia? A. It requires a zone of slow conduction to perpetuate B. It is caused by changes in intracellular calcium concentration C. A mapping strategy using entrainment would like identify an ablation target D. Dofetilide is a good initial therapeutic option
Footnotes
-
Fogoros, R. N., & Mandrola, J. M. (2017). Fogoros’ electrophysiologic testing. John Wiley & Sons. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9 ↩10 ↩11 ↩12 ↩13
-
Al-Khatib, S. M., Stevenson, W. G., Ackerman, M. J., Bryant, W. J., Callans, D. J., Curtis, A. B., … & Page, R. L. (2018). 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology, 72(14), e91-e220. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7