- Fibrous thickening of the pericardium forms a rigid, noncompliant encasement of the heart, resulting in progressive impairment in cardiovascular filling and low cardiac output.
- The pericardium is thick and can’t stretch (less compliant) during diastole, i.e. impaired diastolic filling of the ventricles due to pericardial disease
- constriction limits the total volume of blood that can be accommodated by the heart during diastole across the respiratory cycle, with equalization of right- and left-sided cardiac filling pressures. 1
- The rigid cardiac encasement isolates the cardiac chambers from swings in intrathoracic pressure
- Inspiration → ↓ in intrathoracic pressure is not fully transmitted to the cardiac chambers b/c they are encased in the rigid pericardium
- “when you inspire, the heart normally drinks”
- Kussmaul Sign
- During expiration, IVC flow competes with SVC flow. The diaphragm squeezes the abdomen → increased IVC flow.
- IVC flow pushes blood up into RA, impedes SVC flow coming into RA → pushes blood up into neck veins → Kussmaul’s sign
- Increase in JVP with inspiration
- tl;dr: Inspiration → Increased IVC flow + Increased SVC flow → flowing into high pressure RA → increased JVP w/ inspiration
- Respiratory variation and Interventricular dependence
- Inspiration reduces intrathoracic pressure which usually is fully transmitted to intracardiac pressures, but in constriction, the intracardiac pressures falls much less than intrathoracic pressure because of pericardial constraint. This difference in pressure change with inspiration results in reduced filling to left side of the heart. The reduction in left heart filling during inspiration causes a reduction in mitral inflow velocity and a shift of the interventricular septum toward the left ventricle. With expiration, left heart filling increases which shifts the interventricular septum back toward the right ventricle, leading to reduced filling to right side of the heart and a late-diastolic reversal of flow in the hepatic veins. 2
- Interdependence of ventricular filling on inspiration: intrathoracic pressure and pulmonary venous pressure ↓, but LA pressure does not. A reduced pulmonary vein to LA pressure gradient results in decreased flow into the LA and LV. Decreased LV filling during diastole allows for increased RV filling, which leads to an increase in flow across the tricuspid valve. Thus on inspiration, RVSP increases while LVSP decreases. On expiration, increased LV filling occurs at the expense of RV filling and the opposite effect occurs on mitral valve and tricuspid valve flow.
“Normally, with inspiration, you’re going to have a drop in your intrathoracic pressures. And typically, those pressure changes are transferred fairly well to the cardiac chambers as well. What happens with constriction is you have this really stiff, non-compliant pericardial sac that serves to somewhat insulate the heart from those normal changes. So essentially what happens is with inspiration, the pulmonary pressures are going to drop (and consequently the pulmonary vein pressures), but that stiff pericardium insulates the LV and the RV. So they don’t really see those pressure changes and the pressure stays relatively constant. So what happens is the driving pressure between what we measure in the cath lab, the pulmonary capillary wedge pressure and the LVEDP is going to vary with respiration. So essentially, with inspiration, you see a decrease in your left ventricular filling and an increase in your right ventricular filling (discordance). And you mentioned the bowing of the septum that we see on the echo, and that’s indicative of the increased RV filling. And then you have the exact opposite with expiration, where you have increased left ventricular filling and the septum bows back into the RV.” - CardioNerds Episode 58
“When I drop my diaphragm, air comes into my thorax. So too, blood comes into my thorax as well. And so normally both the RV and the LV will fill and they’re not competing against each other because the RV could accommodate all that fluid by bowing out into the pericardium, which is supposed to be soft and accommodating. And so the RV drinks and the LV could drink and it’s not a tug of war. But when you have this heart locked in this cage, a steel cage, if you want to think about it, or just a really calcified cage, for example, where the heart is, as you said, totally insulated from the changes in thoracic pressure, the pulmonary vessels are actually outside of the pericardium so they are not subjected. The IVC and the SVC are also outside of that cage. They basically are feeling this negative pressure and trying to bring in fluid. And as that RV fills up and it doesn’t have that free wall to bow out and accommodate all that extra fluid, it actually shoves the septum over into the LV and causes the LV to suffer while the RV is taking advantage of this increased flow. And so you end up having discordance between the cardiac output going out of the RV and the cardiac output going out of the LV when normally they’re supposed to equal each other. And so that’s what ends up giving you this shift in inflow velocities through the tricuspid valve, which again is the fluid coming through the tricuspid valve is going to be different during inspiration than during expiration when there is a shift back now. And now the LV says, “ha! it’s my turn.” And it shoves the septum back into the RV. And so now there’ll be more of an increased flow into the mitral valve.”
- CardioNerds Episode 58
- More commonly will follow bacterial pericarditis
- risk of progression is especially related to the etiology: low (<1%) in viral and idiopathic pericarditis, intermediate (2-5%) in immune-mediated pericarditis and neoplastic pericardial diseases and high (20-30%) in bacterial pericarditis, especially purulent pericarditis
Equalization of diastolic pressures are non-specific
The traditional invasive hemodynamic criteria of “equalization of end-diastolic pressures” is not specific for constrictive pericarditis. (Source)
-
Classic clinical picture: Si/Sx of right heart failure and impaired diastolic filling d/t pericardial constriction with preserved right and left ventricular function in the absence of previous or concomitant myocardial disease or advanced forms.
- Can have hemodynamic impairment if systolic dysfunction d/t myocardial fibrosis or atrophy (more advanced cases)
-
History: fatigue, peripheral edema, breathlessness and abdominal swelling
-
⚠️ Although classic and advanced cases show prominent pericardial thickening and calcifications in chronic forms, constriction may also be present with normal pericardial thickness in up to 20% of the cases
-
Respirophasic hemodynamic augmentation
- Expiration → rise in intrathoracic (and ∴ pulmonary venous) pressures → ↑ flow into the L heart → b/c it is within a fixed total intrapericardial volume, the increased L heart filling pushes the IV septum towards the right → reduced RV filling → expiratory diastolic flow reversals are transmitted back to the IVC and hepatic veins.
-
Main DDx is restrictive cardiomyopathy
- Constrictive pericarditis (CP) is a potentially reversible cause of heart failure, whereas restrictive cardiomyopathy (RCM) has very limited therapeutic options. 1
- Restrictive cardiomyopathy is primarily a disease of the myocardium and is characterized by increased myocardial stiffness, which results in a rapid rise in ventricular filling pressures reflected in both the systemic and pulmonary circulations 1
- Abnormal diastolic dysfunction, but preserved LVEF
- Amyloidosis is the most common secondary form of RCM
- Treatment is often limited to heart transplantation
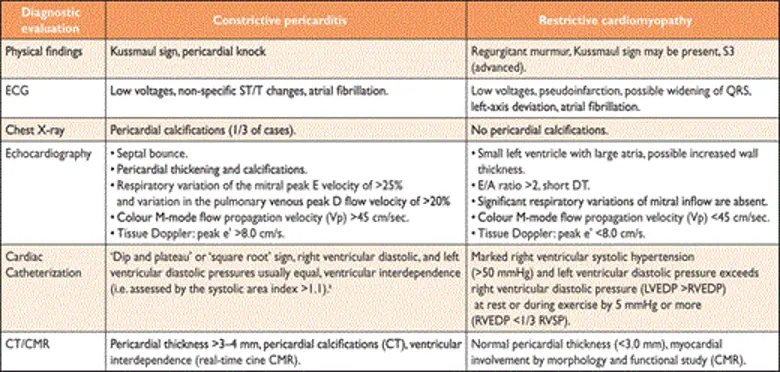
- The utility of CMR in constrictive pericardial disease is well established, providing the opportunity not only to evaluate pericardial thickness, cardiac morphology and function, but also for imaging intrathoracic cavity structures, allowing the differentiation of constrictive pericarditis from restrictive cardiomyopathy. Assessment of ventricular coupling with real-time cine magnetic resonance during free breathing allows an accurate evaluation of ventricular interdependence and septal bounce.
Constriction versus Restriction
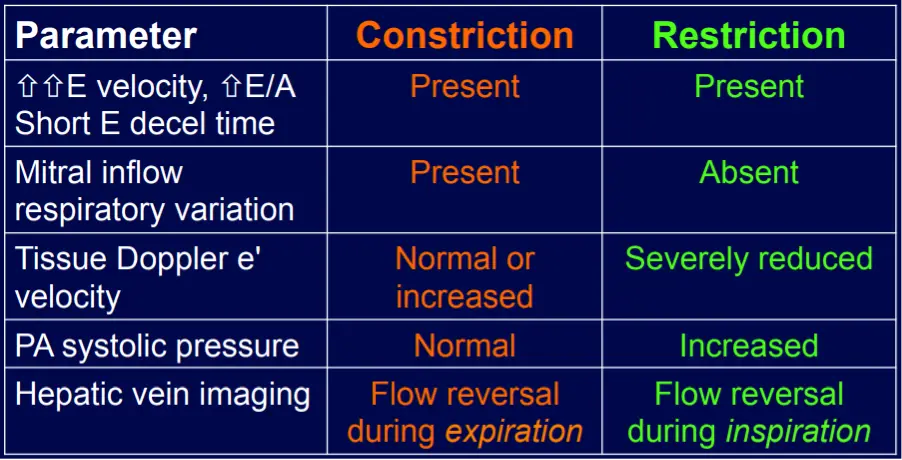
- Both share a common clinical presentation of predominantly right-sided heart failure, in the absence of significant left ventricular systolic dysfunction (i.e., HFpEF) or valve disease, due to impaired ventricular diastolic filling. 1
- Constrictive pericarditis will feature End-diastolic pressure equalization (typically within 5 mmHg) between these cardiac chambers because of the fixed and limited space within the thickened and stiff pericardium.
- PASP are usually normal in constrictive pericarditis; higher pulmonary pressures suggest a **restrictive cardiomyopathy
- Echo parameters are better at differentiating constriction vs restriction than BNP (Sengupta AJC, 2008)
- Postulated that BNP would be decreased in patients with CP despite increased left ventricular filling pressures because the constrained pericardium would prevent myocardial stretch despite increased filling pressures. However, most present patients with CP are older and have secondary causes, such as previous cardiac surgery and radiotherapy, which may be associated with some concomitant myocardial disease
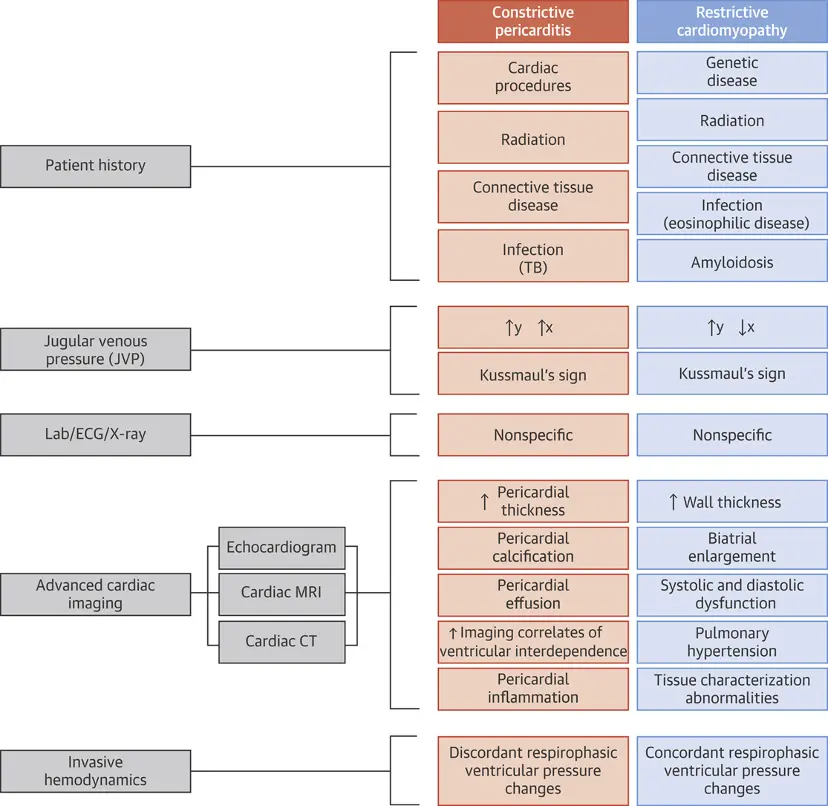
Flavors of constriction
- Chronic constriction
- Transient constrictive pericarditis
- usually develops with pericarditis and mild effusion and resolves with anti-inflammatory therapy within several weeks
- Constriction is d/t inflammation → resolves w/ Tx of the inflammation
- Effusive-constrictive pericarditis
- pericardial cavity is typically obliterated, i.e. even the normal amount of pericardial fluid is absent
- Scarred pericardium 1) constricts cardiac volume and 2) can put pericardial fluid under increased pressure (→ signs of cardiac tamponade)
- Causes include idiopathic, radiation, neoplasia, chemotherapy, infection (especially TB and purulent forms) and post-surgical pericardial disease
- Clinical features of pericardial effusion or constrictive pericarditis, or both
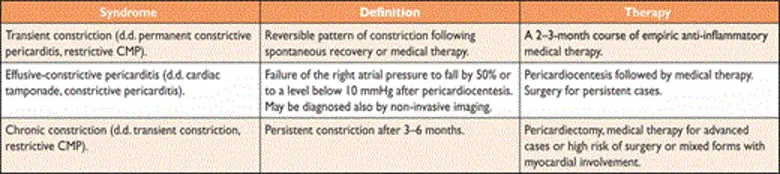
Diagnosis
- Echo, CT (with contrast enhancement to assess pericardial inflammation), CMR, Cath
- Since the inflamed pericardium is enhanced on CT and/or CMR, multimodality imaging with CT and CMR may be helpful to detect pericardial inflammation. Can be useful to see if you’re dealing with transient constrictive pericarditis.
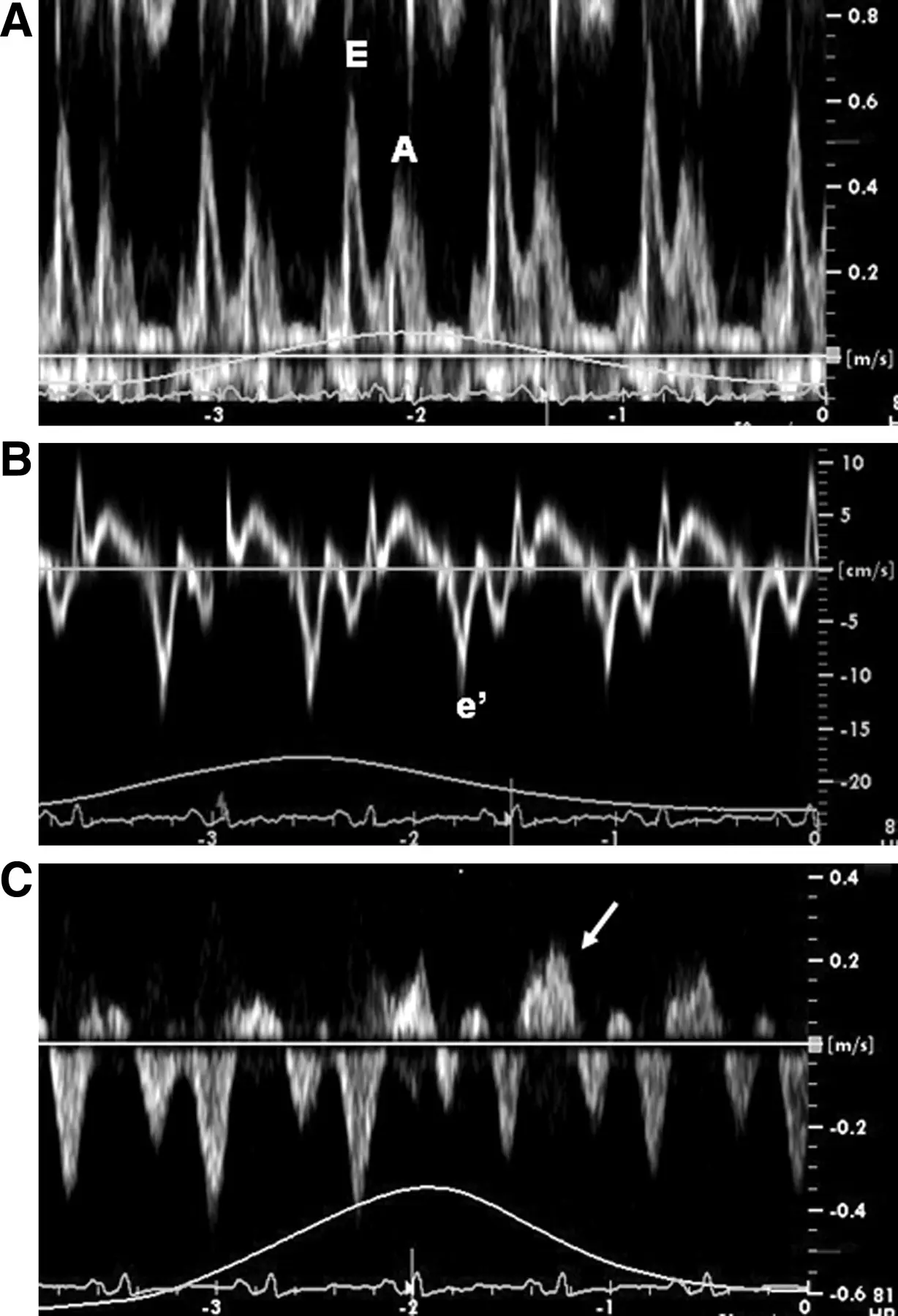
- Specific diagnostic echocardiographic criteria for the diagnosis of constrictive pericarditis has been proposed by the Mayo Clinic and include:
- septal bounce or ventricular septal shift with either medial e′ >8 cm/s or hepatic vein expiratory diastolic reversal ratio >0.78 (sensitivity 87%, specificity 91%; specificity may increase to 97% if all criteria are present with a correspondent decrease of sensitivity to 64%
- ‘End-stage’ constrictive pericarditis
- Manifestations include cachexia, atrial fibrillation, a low cardiac output (cardiac index <1.2 l/m2/min) at rest, hypoalbuminemia due to protein-losing enteropathy and/or impaired hepatic function due to chronic congestion or cardiogenic cirrhosis.
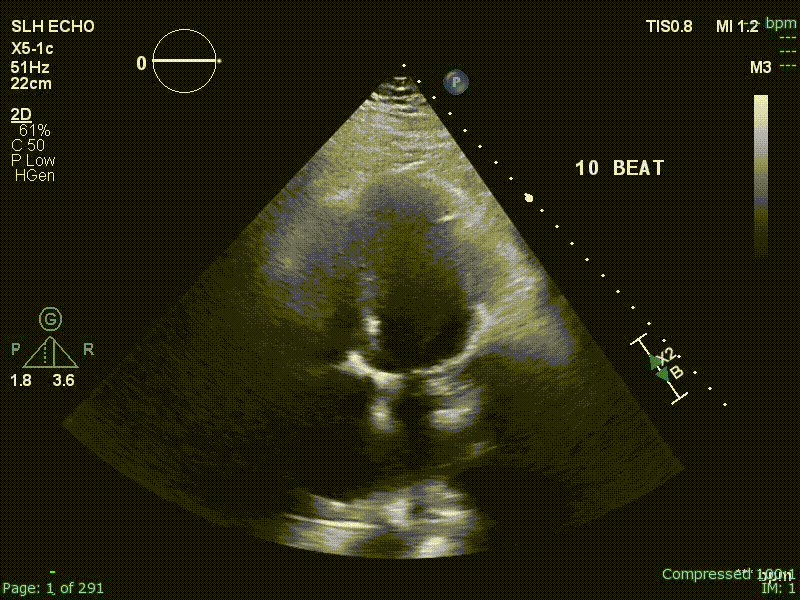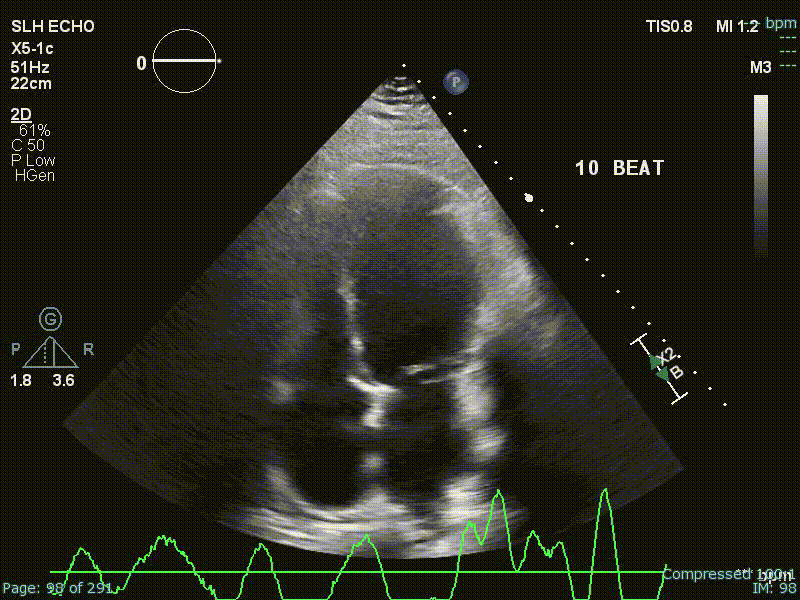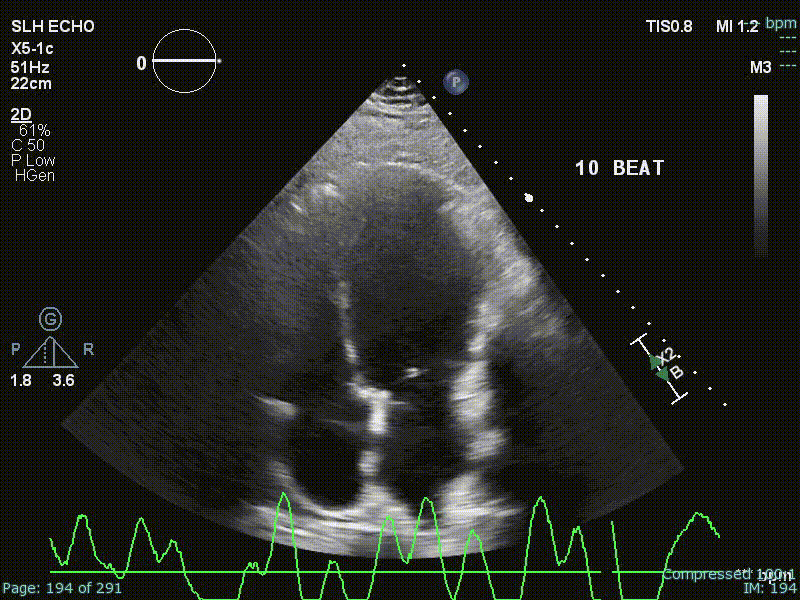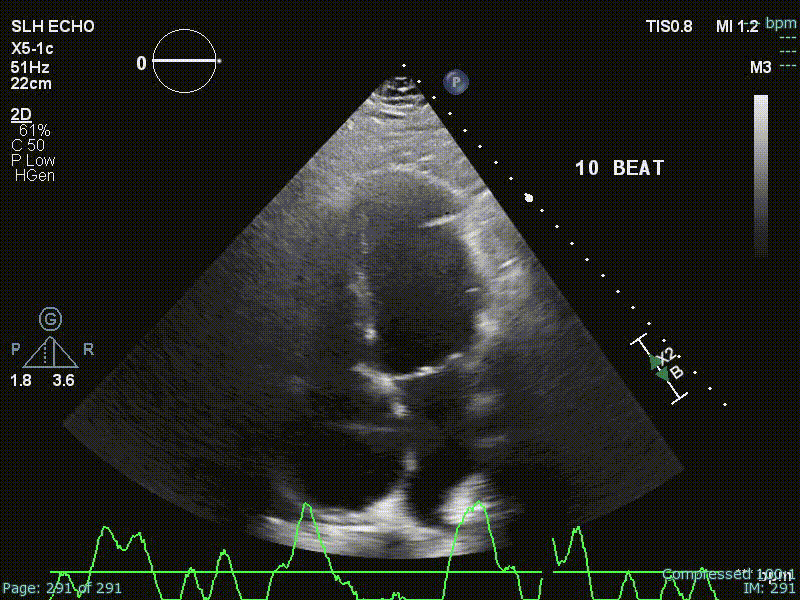
Echo
“Echo diagnosis of constriction is all about pattern recognition” - Sanjiv Shah
Ventricular Interdependence? Think constriction
Constrictive physiology should be considered when evidence of enhanced interdependence is observed by echocardiography.
- Combined parameters from mitral inflow, mitral annular velocities, and hepatic vein velocities have made the diagnosis of constriction and its differentiation from myocardial disease much easier than ever before.3
- Three variables were independently associated with constrictive pericarditis: 1) the presence of ventricular septal shift, 2) medial mitral e’ velocity; and 3) the hepatic vein expiratory diastolic reversal ratio. 2
- Each of these criteria was also significantly associated with constrictive pericarditis in the subset of patients with atrial fibrillation or flutter.
- The presence of ventricular septal shift in combination with either medial e’ ≥ 9 cm/s or hepatic vein expiratory diastolic reversal ratio ≥ 0.79 (Hepatic vein diastolic reversal velocity / diastolic forward flow velocity) was 87% sensitive and 91% specific for the diagnosis of constrictive pericarditis.
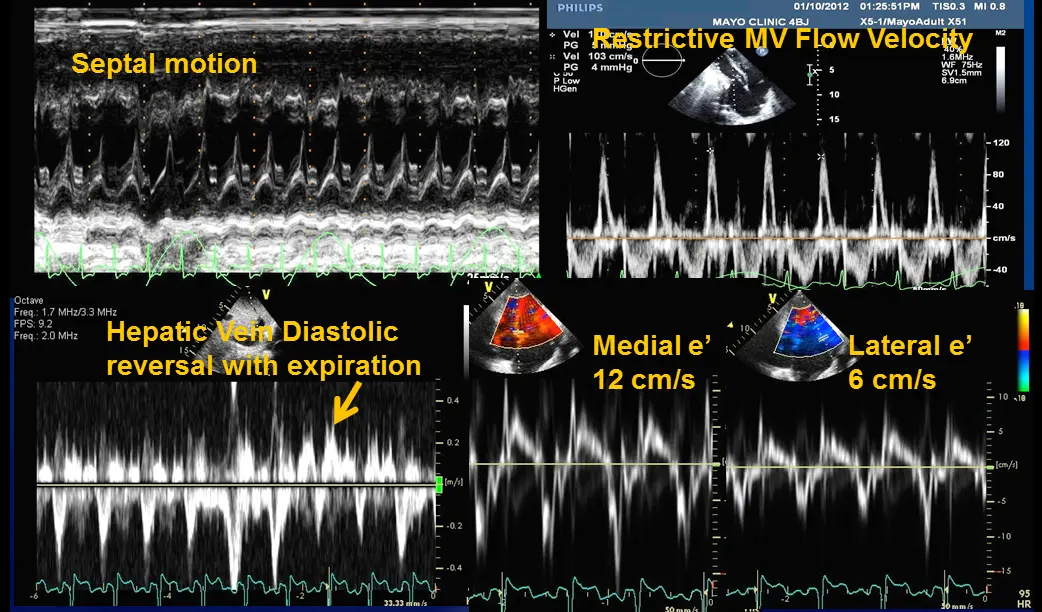
- Diastolic septal bounce
- Ventricular interdepence and Respirophasic Variation
- Expiration: mitral inflows ↑, tricuspid inflows ↓
- “When you expire, you increase the pressures in the thorax, but that doesn’t transmit to the LV. And so you have an increased drive from the pulmonary veins to go into and fill the left side. And so in expiration, your mitral inflow goes up. But because of that ventricular interdependence, your tricuspid inflow goes down. And so you have this respirophasic variation in mitral inflow going up with expiration, tricuspid inflow going down with expiration, and then vice versa with inspiration. But for the same exact reason, you get respirophasic shifts in the septum. And so if you do an M-mode through the PLAX view, you’ll see the septum globally shifting towards the right side because the LV is filling during expiration and then shift back towards the left side because the RV is preferentially filling better during inspiration.”
- Expiration: mitral inflows ↑, tricuspid inflows ↓
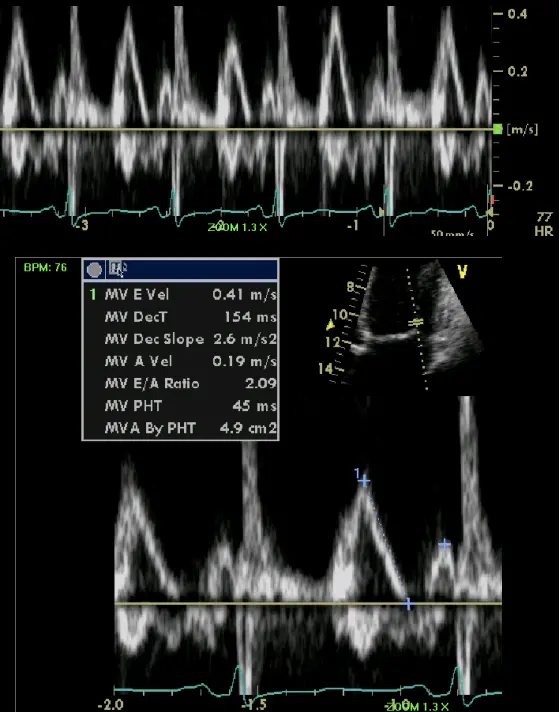
- Mitral inflow ↑ respiratory variation, ↑ E/A, ↓ E deceleration time
- Preserved e’ velocity (septal ≥ lateral)
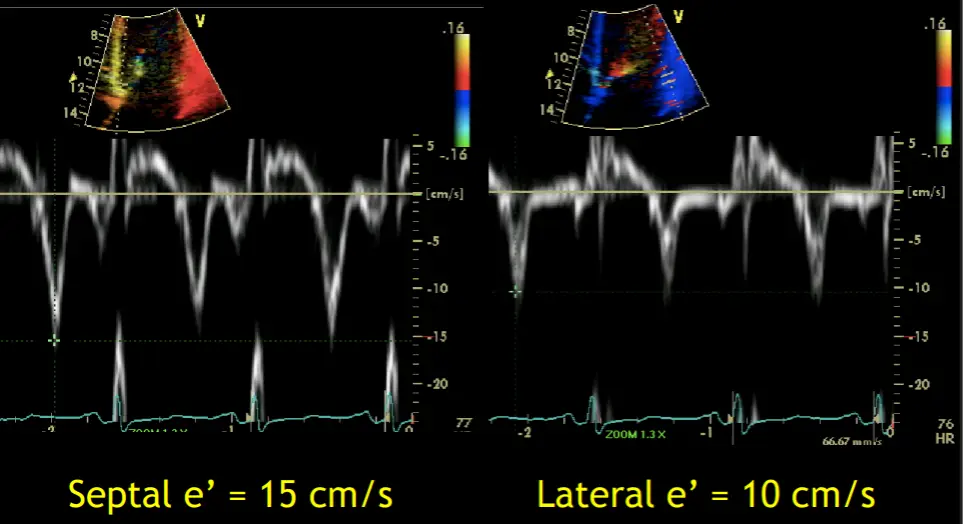
- By contrast, with restrictive physiology you will expect reduced e’ velocities
- “Your e’ essentially is the tissue velocity of the mitral annulus. And you can look at the medial e’ and the lateral e’. And by definition, restrictive heart disease, there’s a stiffening of the muscle and it doesn’t relax as well. And so the movement of the muscle is low. And so you’ll have a patient with heart failure, preserved ejection fraction, predominant right-sided symptoms, and your e’ will be low, as you suspect, because there’s diastolic dysfunction. However, with constriction, it’s not a muscle disease. And so you may have normal or elevated e’ velocities in the same clinical context or preserved EF. right-sided symptoms with elevated filling pressures. And so normal or elevated e’ tissue Doppler velocities of the mitral annulus can help promote that it’s constriction and not restriction. And alternatively, with the E-prime velocities, you can compare the medial e’ velocity to the lateral e’ velocity. And the normal healthy heart, the septum is a little bit restricted because it’s tethered to the cardiac skeleton. And so normally the lateral e’ has a greater velocity. The lateral mitral annulus moves faster than the medial mitral annulus. But in constriction, because of the tethering of the lateral wall to the constricted, inflamed, fibrous pericardium, you’ll have annulus reversus whereby the medial e’ velocity will be greater than the lateral e’ velocity.”
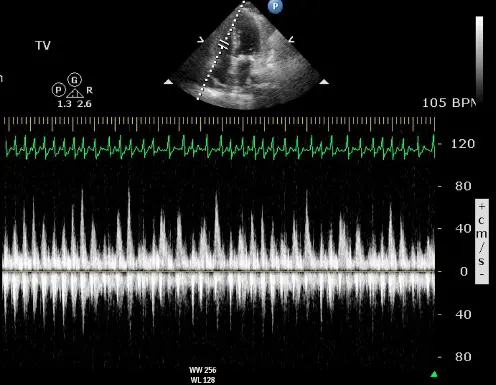
- Dilated IVC
- Diastolic flow reversal during expiration
- Hepatic vein flow reversal increases with expiration
- “The hepatic vein doppler is very helpful in atrial fibrillation in patients with constrictive pericarditis to differentiate them from restrictive physiology. And so if you think about hepatic inflow, the hepatic vein should be draining into the IVC and then into the right atrium. And so if a patient has constrictive pericarditis, what happens with expiration? That septum’s blowing towards the right side, and that pressure is being transmitted from the RV to the RA to the IVC back up to the hepatic veins. So that you have ==diastolic flow reversal in hepatic veins predominantly during expiration== if the patient has constricted pericarditis. It’s all along the same track from the LV to the RV to the RA, IVC, and hepatic veins.
- Conversely, if a patient has restrictive physiology, what happens is the majority of the inflow to the right side, just like in normal healthy hearts, will happen during inspiration. However, the restrictive, stiff, non-compliant right ventricle, it’s not able to fully accept all that blood during inspiration. And so during inspiration, when you get that huge bolus going into the RV, it’s not going to be able to accept it. And so you get the pressure referred all the way backwards into the hepatic veins. And so ==with the restrictive physiology, you get diastolic flow reversal in the hepatic veins, predominantly in inspiration.==”
- Reduced radial function, preserved longitudinal function
Tissue Doppler
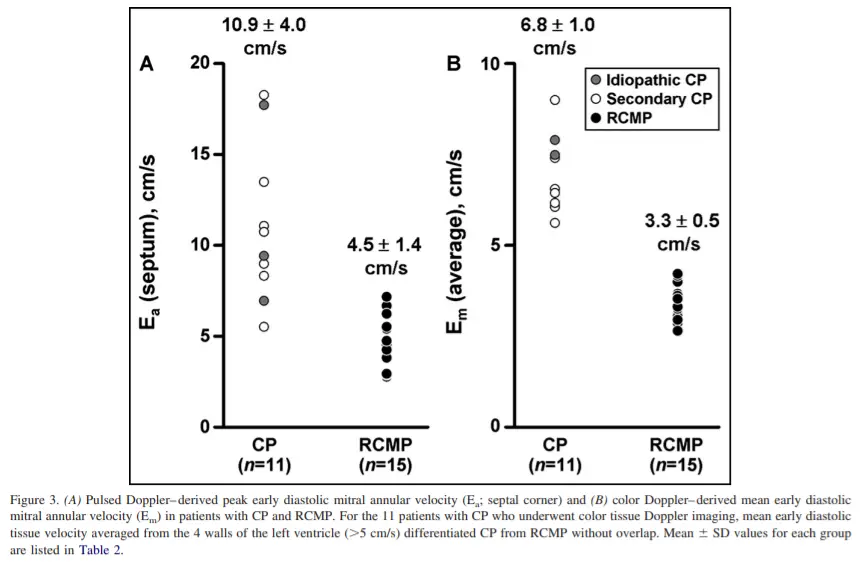
- Rationale: Because the mechanoelastic properties of the myocardium are preserved in patients with constrictive pericarditis, longitudinal early diastolic myocardial velocities are higher than in patients with Restrictive Cardiomyopathy.
- “A mean annular velocity (averaged from 4 walls) 5 cm/s correctly distinguished CP from RCMP, even when there was a large overlap of BNP between the 2 groups.” (Sengupta AJC, 2008)
Hemodynamics
- Elevation of and equalization of diastolic pressures are the hallmarks of constriction.
- RVEDP and LVEDP will be within 5 mmHg of each other.
- In contrast to the normal heart, where pressures in the cardiac chambers are independent (i.e. unrelated) during diastole, in constrictive pericarditis, the stiff pericardium limits expansion of the cardiac chambers. The chambers can fill beyond a certain limited point only by compressing other chambers, and thus the diastolic pressures equalize.
- Simultaneous RHC + LHC to get RV and LV pressure tracings simultaneously
- Hemodynamic findings in CP
- Almost all ventricular filling occurs in early to mid-diastole
- ↑ atrial pressures
- Equalization of early and mid-diastolic pressures (e.g., RV and LV or RA and LV)
- ↑ in RA pressure during inspiration (Kussmaul’s sign)
- Exaggerated X and Y descents
- Intrathoracic pressure is not transmitted to the cardiac chambers
- Occurs due to the effects of respiration on ventricular filling.
- Ventricular discordance
- Accentuated rapid ventricular filling
- d/t high atrial driving pressures and unimpeded ventricular relaxation
- Followed by rapid “y” descent, which represents sudden rapid rise in pressure from pericardial restraint
- Exaggerated “x” descent d/t an exaggerated ventricular longitudinal contraction
- ==High diastolic pressures with a paradoxically low SV d/t low preload.==
- Classic appearance:
- Rapid “y” descent on atrial pressure waveform
- “Square root” sign on ventricular pressure waveform
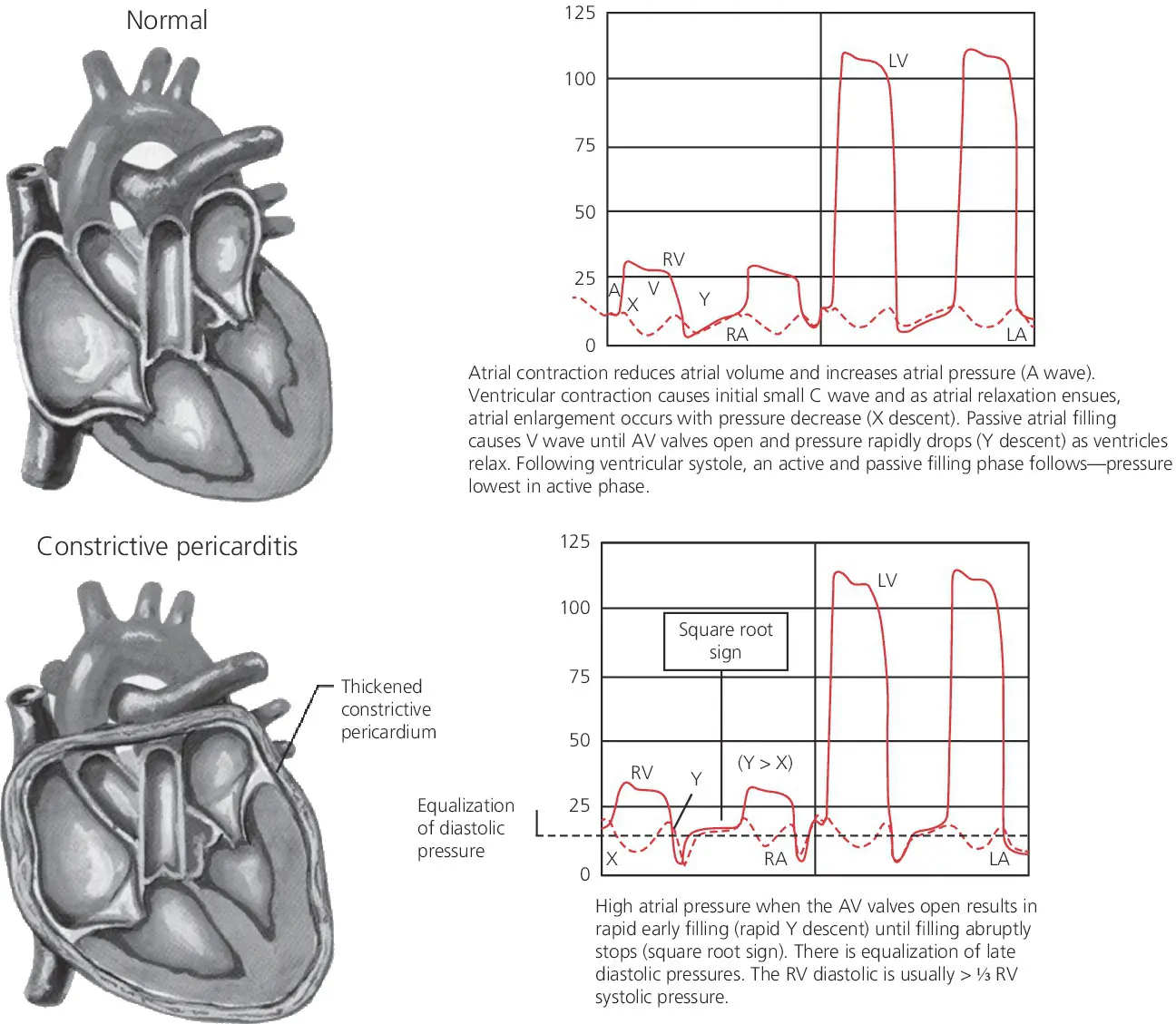 Figure source
Figure source
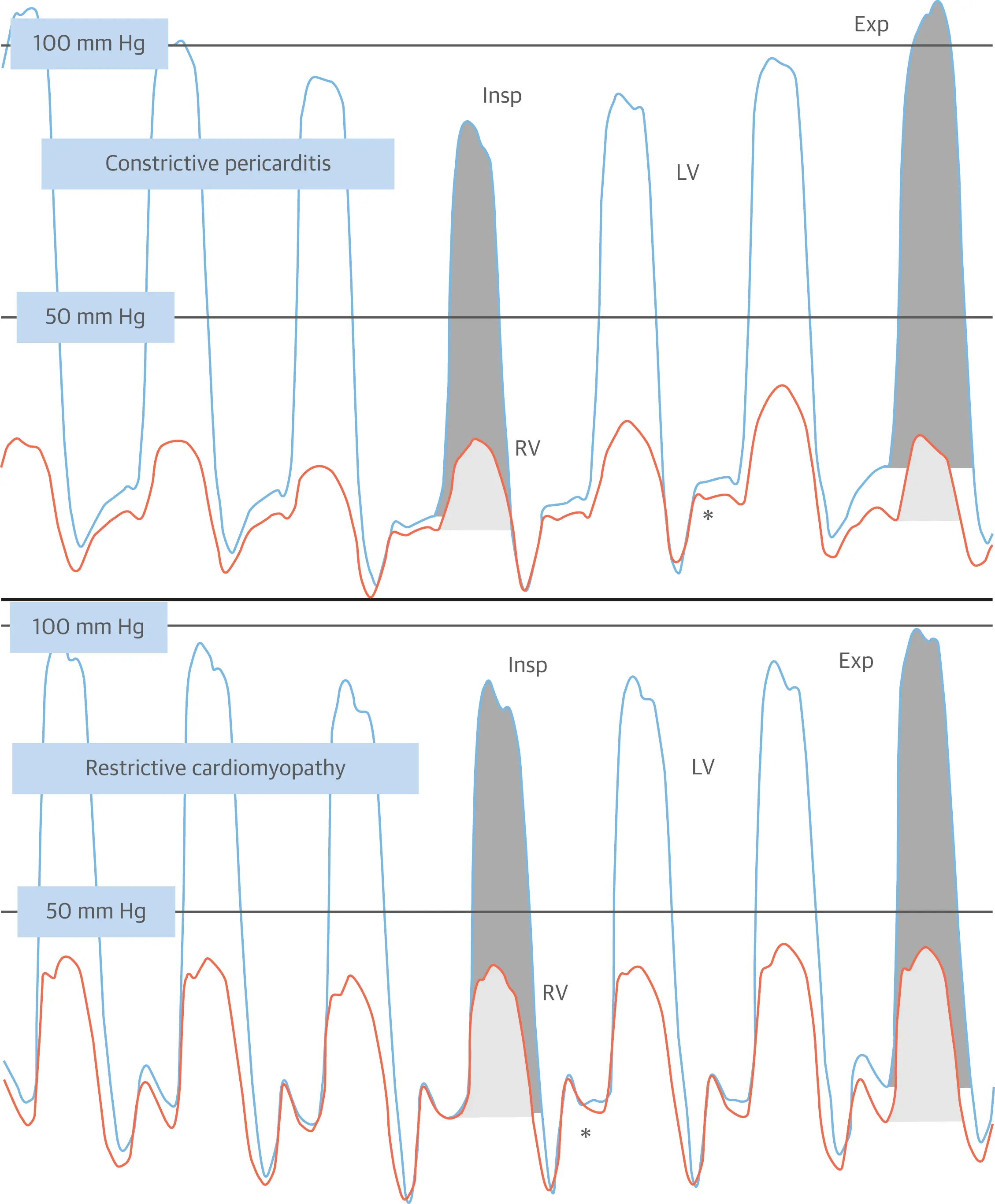
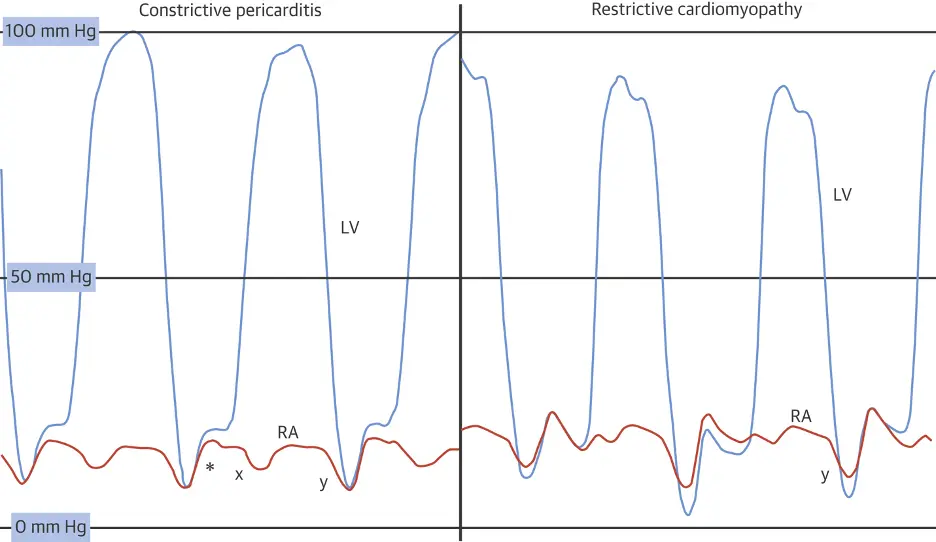 Caption: (Left) Left ventricular (LV) (blue) and right atrial pressure (RA) (orange) hemodynamic pressure tracings in constrictive pericarditis (CP). Prominent “x” and “y” descents are present with a square root sign (*). (Right) LV and RA pressure hemodynamic pressure tracings in restrictive cardiomyopathy (RCM). A prominent “y” descent is present, but the “x” descent is blunted. (Figure source: 1)
Caption: (Left) Left ventricular (LV) (blue) and right atrial pressure (RA) (orange) hemodynamic pressure tracings in constrictive pericarditis (CP). Prominent “x” and “y” descents are present with a square root sign (*). (Right) LV and RA pressure hemodynamic pressure tracings in restrictive cardiomyopathy (RCM). A prominent “y” descent is present, but the “x” descent is blunted. (Figure source: 1)
Management
- 📝 in the absence of evidence that the condition is chronic (e.g. cachexia, atrial fibrillation, hepatic dysfunction or pericardial calcification), patients with newly diagnosed constrictive pericarditis who are hemodynamically stable may be given a trial of conservative management for 2—3 months before recommending pericardiectomy. This is b/c it may be transient constrictive pericarditis and could resolve with Tx.
- Surgery
- Pericardiectomy is the accepted standard of treatment in patients with chronic constrictive pericarditis who have persistent and prominent symptoms such as NYHA class III or IV.
- Patients with ‘end-stage’ constrictive pericarditis derive little or no benefit from pericardiectomy, and the operative risk is inordinately high.
- Predictors of poor overall survival are prior radiation, worse renal function, higher pulmonary artery systolic pressure, abnormal left ventricular systolic function, lower serum sodium level and older age.
- 📝 Pericardial calcification had no impact on survival.
- Complete surgical removal of the pericardium can result in excellent symptomatic improvement. The prognosis is dependent upon the underlying etiology, with prior radiation therapy consistently associated with worse outcomes
- Pericardiectomy is the accepted standard of treatment in patients with chronic constrictive pericarditis who have persistent and prominent symptoms such as NYHA class III or IV.
- Medical therapy has a role in 3 cases:
- Specific etiology, such as TB pericarditis, to prevent progression to constriction
- Transient constriction d/t pericarditis — gets better as you Tx the pericarditis
- Supportive — control Sx of congestion in advanced cases or when surgery contraindicated/high-risk
Footnotes
-
Geske JB, Anavekar NS, Nishimura RA, Oh JK, Gersh BJ. Differentiation of Constriction and Restriction. Journal of the American College of Cardiology. 2016;68(21):2329-2347. doi:10.1016/j.jacc.2016.08.050 ↩ ↩2 ↩3 ↩4 ↩5
-
https://www.acc.org/Latest-in-Cardiology/Articles/2015/03/09/07/22/Mayo-Clinic-Echocardiography-Diagnostic-Criteria-for-Constriction ↩ ↩2
-
Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011 Jul;4(4):444-55. doi: 10.1161/CIRCIMAGING.110.961623. PMID: 21772012. ↩